-
07/14/25: High Temperatures coming to Washington
OLYMPIA – The National Weather Service is forecasting dangerously high temperatures across parts of Washington this week. Heat advisories are issued for several counties statewide for much of the week, with dangerously high temperatures both during the day and overnight and close to 100 degree highs expected Wednesday in some areas. The National Weather Service is High heat can…
-
07/01/25 Health Advisory: Screening guidance for Candida auris in light of new local cases
Summary: Upgrade screening and preparation for Candida auris. Two facilities in Seattle have known transmission of Candida auris (C. auris). We have limited information about how much transmission is occurring in Washington. We recommend that you: Early screening is critical for rapid identification so you can quickly implement infection control measures and prevent spread at your facility. Background As of…
-
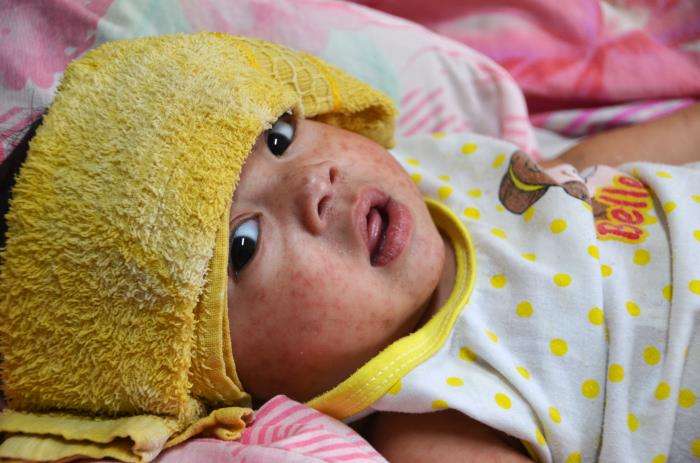
04/18/25 Health Advisory: Stay alert for measles cases
Summary: Recognize measles and notify the Health Department. Consider measles as a diagnosis in anyone who: If you suspect measles, call the Health Department before the patient leaves the clinic using . Background There is a large, ongoing measles outbreak in Texas. Several other states have reported smaller outbreaks of measles. Here in Washington, 4 cases have…
-
03/10/25 Health Advisory: Report any serious adverse events after ceftriaxone administration
Summary On Feb. 7, 2025, Centers for Disease Control and Prevention (CDC) issued a call for cases related to about 10 severe adverse events that closely followed ceftriaxone injection or infusion. The events began Sept. 1, 2024. No single product type or lot number is tied to these severe events. While there is no confirmed…
-
Provider Alert: Subtyping of Influenza A is Recommended for Hospitalized Patients
This is a Provider Alert from the Washington State Department of Health and . On January 16, 2025 the Centers for Disease Control and Prevention (CDC) issued a Health Advisory recommending influenza A testing for all hospitalized patients with suspected influenza, and also recommending expedited subtyping for influenza A-positive specimens. Providers and clinical laboratories in…
-
10/23/24 Health Advisory: Presumed positive human cases of avian influenza in Washington.
Washington State Department of Health is investigating presumed positive human cases of avian influenza in Washington. Healthcare providers should consider avian influenza in patients who present with acute respiratory illness, isolated conjunctivitis, or influenza-like illness (ILI). Assess patients for exposure to animals––especially poultry, cattle, and wildlife, or to people suspected or known to be infected. Current…
-
10/17/24 Health Advisory: Disruptions in Availability of Peritoneal Dialysis and Intravenous Solutions from Baxter International Facility in North Carolina
BackgroundOver several days in late September 2024, Hurricane Helene caused extensive damage to the southeastern United States. The storm affected the Baxter International’s North Cove facility in North Carolina, the largest manufacturer of peritoneal dialysis and intravenous solutions in the United States, halting production. Additional supply disruption may occur in the aftermath of Hurricane Milton. Recommendations for Healthcare…
-
09/23/24 Health Advisory: 2024–2025 Respiratory Illness Season Vaccine Recommendations
Summary COVID-19 vaccine recommendations For most people, the minimum interval between their last 2023–2024 COVID-19 vaccine dose and their 2024–2025 COVID-19 vaccine dose is 8 weeks. There are exceptions for people completing a multidose initial vaccination series, including: Learn more in CDC’s detailed vaccination schedules for people who are not moderately or severely immunocompromised and people who are…
-
05/23/24 Health Advisory: Pertussis Increasing in Washington
Summary Current situation in Washington In 2023 CDC (Centers for Disease Control and Prevention) week 1–18, healthcare providers reported 24 cases of confirmed or probable pertussis in Washington. In this same period in 2024, providers reported 170 cases. This is a 6-fold increase. Multiple health jurisdictions reported increases. DOH (Washington State Department of Health) updates its weekly pertussis update every Friday. Recommendations for…
-
04/30/24 Health Advisory: Invasive Serogroup Y Meningococcal Disease Increasing in the United States
Summary Invasive serogroup Y meningococcal disease cases are on the rise in the United States. Cases are disproportionately occurring in people who are: 30–60 years old. Black or African American. Living with HIV. Washington has not seen an increase in cases. Patients with invasive meningococcal disease may have bloodstream infection or septic arthritis, without typical meningitis symptoms…
-
03/27/2024 Health Advisory: Measles Increasing in Washington, the Nation, and the World
Summary Background Measles is a highly contagious viral illness that can lead to severe health complications, like pneumonia, encephalitis, and death, especially in unvaccinated people. Measles outbreaks have occurred in Washington, the United States, and around the world in 2024. Outbreaks often start when an unvaccinated or under-vaccinated person is exposed during international travel and…
-
02/08/24 Health Advisory: RSV Prevention Updates
Summary Background On Oct. 23, 2023, Centers for Disease Control and Prevention (CDC) issued a health alert about the limited supply of nirsevimab. CDC recommended healthcare providers prioritize nirsevimab for infants born later in RSV (respiratory syncytial virus) season or for those at increased risk for severe illness. On Jan. 5, 2024, CDC issued updated guidance about the increased…
-
01/19/24 Health Advisory: Measles Outbreak in Southwest Washington
An active measles outbreak investigation is underway in Clark and Wahkiakum Counties. As of Jan. 10, 2024, investigators have identified 3 lab-confirmed and 3 epidemiologically linked measles cases in unvaccinated adults. Symptoms began in mid-to-late December. All report isolating at home during their contagious period. Investigators have not identified any public exposure locations. Healthcare providers…